Under the new medical reform policy, 3 categories of drugs to meet the development! Fee control becomes the norm, and pharmaceutical companies have 3 ways out.
Release time:
2020-05-06
Wonderful content
In recent years, policies covering the fields of medicine, medical treatment, medical insurance and circulation have been intensively released, such as the consistency evaluation of generic drugs in the pharmaceutical industry, the release of the national key monitoring catalogue, the cancellation of drug addition and graded diagnosis and treatment in the medical industry, the reform of medical insurance payment mode, the adjustment of medical insurance catalogue, and the two-vote system and business tax reform in circulation, all have a profound impact on the development of the pharmaceutical industry.
On November 9, the Guangdong-Hong Kong-Macao Friendship Association of China Pharmaceutical University and the Guangdong Pharmaceutical Association held the second "Southern Medicine Summit Forum". In the morning, the research and development sub-venue, the quality sub-venue and the marketing sub-venue took the lead. The research and development sub-venue mainly introduced high-end preparations such as inhalants, slow-release agents, skeleton tablets, improved new drugs, innovative drugs and other related contents. The quality sub-venue mainly introduces drug quality control, quality system construction and other related contents. The main marketing venue mainly introduces the transformation of marketing strategy in the post -47 era and MAH enterprise national service.
In the afternoon, at the main venue hosted by Zong Yungang, deputy director of the Southern Institute of Pharmaceutical Economics of the State Drug Administration, Zhang Buyong, general manager/chief researcher of Mi Nei, Xu Wei, professor of China Pharmaceutical University, Lin Qiyi, director of the second Drug Supervision and Administration Division of Guangdong Drug Administration, and Zheng Zhihua, vice chairman and secretary-general of Guangdong Pharmaceutical Association, expressed their views on the impact of the pharmaceutical industry from the macroeconomic situation and policies.

The 2nd Nanyao Summit Forum
The pharmaceutical market is expanding steadily, and the grassroots are welcoming the outbreak. Where are the opportunities for pharmacies?
From January to September 2018, the added value of my country's pharmaceutical industry above designated size increased by 10.2 year-on-year, which was 3.80 percentage points higher than the overall growth rate of the national industry. Xu Wei believes that there is a strong correlation between the income and expenditure of the medical insurance fund, the main business income of the pharmaceutical manufacturing industry, and the main business income of listed pharmaceutical companies. Therefore, the future pharmaceutical market will not grow rapidly, but will Maintain steady expansion at a growth rate slightly higher than GDP.
From the perspective of drug terminals, according to the data of Mi Intranet, in 2018, the sales of drugs in China's three major terminals and six major markets reached 1713.1 billion billion yuan, an increase of 6.3 percent over the same period last year. If the uncounted "private hospitals, private clinics and village clinics" are added, the total sales of drug terminals in China will be about 2000 billion yuan. The proportion of public hospital terminals, retail pharmacy terminals and public primary medical terminals was 67.4 per cent, 22.9 per cent and 9.7 per cent, respectively.
Before the universal medical insurance, my country's pharmaceutical market showed an "inverted triangle" structure. The scarce number of tertiary hospitals undertook most of the patient flow, while the large number of primary medical markets were rarely visited. However, with the continuous promotion of volume procurement and the publication of key monitoring catalogues... Under the control of medical insurance fees, public hospitals return to "public welfare", and their growth will slow down the fastest in the three major terminals in the future. In addition, with the announcement of the list of pilot cities for the construction of medical consortia in 118 cities, the implementation of a series of policies such as hierarchical diagnosis and treatment and chronic disease management, the primary medical market has entered a harvest period, and the structure will change in the future, the "positive triangle" structure will be strengthened.
In 2018, the "Internet Medical Health" opinion was issued, online pharmacies ushered in development, and traditional pharmacies faced challenges; Zhejiang medical insurance designated retail pharmacies and medical insurance medical institutions uniformly implemented medical insurance payment limits, and their retail pharmacy market share declined in 2018; prescription outflow The direction of the policy is gradually becoming clear. As of September 2019, 15 provinces and cities across the country have clearly proposed the establishment of provincial prescription circulation platforms... All these have a great impact on the development of retail pharmacies. Zhang Buyong believes that in the future, physical pharmacies need to develop in the direction of minor diseases (common and frequently-occurring drugs), chronic diseases (long-term drugs) and healthy and optimized life (donkey-hide gelatin, ED, vitamins and calcium).
The structure of drug use is changing dramatically, and low-cost drugs, therapeutic drugs and innovative drugs are popular.
Before 2018, the original research drugs that have passed the patent period enjoy "super national treatment" in China. Although there are many generic drug companies grabbing the market, the original research manufacturers still occupy more than 50% of the market share, and the winning price is several times more than that of generic drugs. Some Chinese patent medicines without evidence-based medical evidence also often appear on the best-selling list of drugs. After 2018, the implementation of policies such as the promotion of 4+7 band procurement and expansion of centralized procurement, the release of the national key monitoring catalogue, and the landing of DRGS payment will lead to fundamental changes in China's drug use structure.
Top 20 Brands of Best-selling Drugs in China, 2018
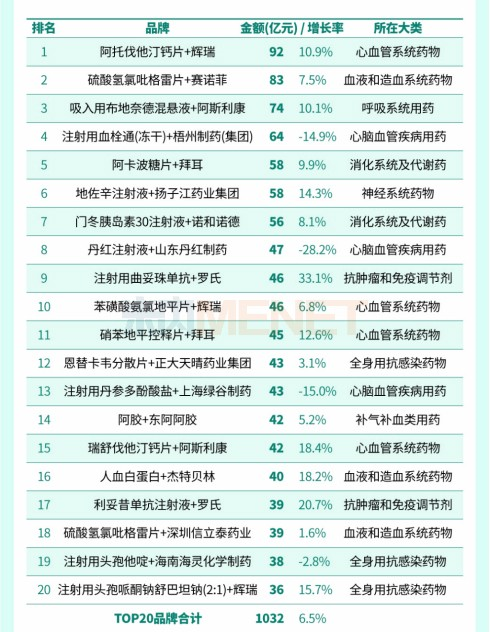
Zhang Buyong believes that in 2018, the world's best-selling TOP100 drugs are mainly innovative drugs and drugs during the patent period, while China's best-selling TOP20 brands are mostly patented original research drugs and proprietary Chinese medicines, which will consume a large amount of medical insurance funds.
The implementation of policies such as the implementation of volume procurement, the release of key monitoring catalogs, and the adjustment of medical insurance catalogs is to make room for space and adjust the structure. The original research drugs and traditional Chinese medicine injections that have passed the patent period will gradually withdraw from the list of best-selling drugs, while the truly therapeutic drugs and innovative drugs will gradually be included in medical insurance, ushering in release. "In the future, drugs related to anti-tumor and autoimmune diseases, drugs for rare diseases and drugs related to neurodegenerative diseases will usher in development opportunities." Zhang Buyong added.
In July 2019, the list of 30 national pilot cities that pay for disease diagnosis-related groupings (DRGS) was announced, and Xu Wei believes that the implementation of DRGS will compress drugs, consumables, inspections and other related costs, and that the structure of drug use in China will change, with relatively low-cost, therapeutic drugs ushering in a positive.
Cost control has become the norm, quality management is the mainstream, pharmaceutical companies have 3 ways out.
Under the new medical reform policy, medical insurance fee control has become the norm. Xu Wei pointed out that price and quantity are the two major factors affecting medical expenses.
The implementation of the two-vote system has purified the pharmaceutical environment, but it cannot achieve the goal of drug price reduction. Therefore, it is necessary to return to the origin of drug recruitment. 4+7 volume purchases have emerged. The prices of 25 varieties have dropped "off a cliff", providing effective measures for price control. The new catalogue of basic drugs optimizes clinical use and limits the growth of medical expenses in quantity. The Opinions on Further Doing a Good Job in Guaranteing Supply and Price Stabilization of Shortage Drugs issued on October 11, 2019 pointed out that the gradual realization of the proportion of essential drugs in government-run primary medical and health institutions, secondary public hospitals, and tertiary public hospitals is in principle No less than 90%, 80%, and 60%, which means that if the company's drugs do not enter the basic drug catalog, it can only squeeze the remaining small amount of market, which has a great impact.
Zheng Zhihua believes that medical insurance control fees are mainly to control the expenses of medical institutions, but if restrictions on doctors' medication lead to bad incidents, this is not in line with the original intention of medical reform. Pharmaceutical care can reduce the incidence of drug damage in patients, thereby reducing the cost of medical insurance.
While controlling fees, we should also emphasize the quality of medical care, and the new drug management law provides policy support for drug quality management. The newly revised Drug Administration Law optimizes the review and approval process, clarifies the division of responsibilities, accelerates the internationalization process with clinical value as the guide, and implements the main body of the holder to reflect the concept of full life cycle supervision. Lin Qiyi pointed out that the new version of the Drug Administration Law will run the effectiveness, safety, and quality controllability of drugs throughout the entire life cycle of drugs to ensure drug quality and safety. The main features include MAH full control, online drug sales online and offline consistency, encourage drug retail chain operation, full traceability, the most stringent supervision.
It can be seen that under the blessing of various policies, pharmaceutical companies are facing severe challenges. Xu Wei provides three ways out: 1. Reduce production costs; 2. Product differentiation; 3. Product innovation. Generic drugs have entered the era of low profit, only through technological improvement, reduce production costs, in order to survive. In the case of fierce competition, differentiated development is more conducive to stand out. Although the road of R & D and innovation is difficult, it has become the main theme of the development of the pharmaceutical industry.






